This section is about how the environmental and health impacts of nitrogen (N) from agri-food systems can be mitigated. It outlines three approaches for mitigating the impacts of agricultural nitrogen (N) use: (1) increasing N use efficiency, (2) spatially redistributing N inputs and agricultural production, (3) and changing human diets.
6.1 Increase nitrogen use efficiency
N use efficiency is defined as the ratio of N in outputs (e.g., meat, milk, or crop harvest) to N in inputs (e.g., feed or fertilizer). For a given amount of outputs, increasing N use efficiency means that the amount going to waste is reduced, as is the need to add new reactive N.
The main principle to keep in mind, before diving into specific efficiency increases, is that a systems perspective is necessary to avoid pollution swapping. For example, if NH3 losses from manure management are reduced, then more manure N is available for application in crops. Unless other crop N inputs are reduced, the increased input of manure N will likely lead to increased leaching or denitrification losses in the field. In general, the idea is that increased efficiency in any part of the system must be matched by increased system-wide production and/ or reduced N input. Otherwise, N losses are merely shifted to another time and place [4,46,136]. Metaphorically, this can be compared to a leaky pipe: emissions depend on the flow through the pipe and the size of holes in the pipe, and the risk is that closing one hole in the pipe causes an increased leak through another [152,153].
Efficiency improvements can be conceptually divided into two categories: (1) increasing conversion efficiency, and (2) increasing circularity. Examples of increasing conversion efficiency are to increase fertilizer N uptake in crops, increase feed use efficiency in livestock, and minimize waste in food and feed processing. Examples of increasing circularity are to increase the use of food waste as livestock feed, and to produce fertilizers from food and feed waste or human excreta. When zooming out to the whole agri-food system, both these categories of improvements ideally lead to reduced waste and a reduced need to input new reactive N whether from synthetic fertilizers or BNF.
On the level of the whole food system, shifting diets to more plant-based food is a major opportunity for increased efficiency. This is discussed separately in Section 6.3 below.
The remainder of this subsection outlines several key principles and technologies for efficiency improvements in the agri-food system.
Crops make optimal use of N inputs when they are healthy and undisturbed by competition and other resource constraints. A large number of approaches and techniques can be combined to ensure healthy and productive crops. Co-limitation of water and other nutrients can be avoided using irrigation, suitable tillage methods, and balanced fertilization [154,155]. Crop diseases, pests, and weeds can be suppressed using a variety of methods, each with their pros and cons: adoption of good crop rotations, promotion of natural enemies, tillage, weeding, and the application of pesticides [81,156,157].
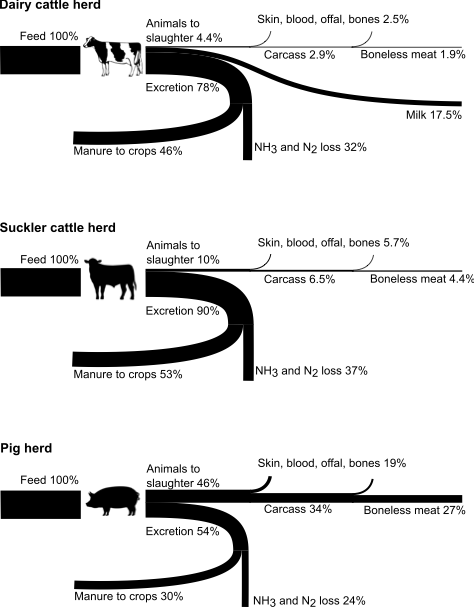
Similarly, livestock are most productive when they are healthy and free from excessive heat, cold, and other stresses. Among many factors, nutritionally balanced feed is one key to livestock health and productivity [45,46]. Also, as mentioned in Section 3, efficiency gains in animal production systems have been reached through breeding and feed formulations that promote faster growth and shorter lives of animals, which leads to a smaller share of feed used for metabolic maintenance. It is important to note, however, that this approach can impact negatively on animal welfare.
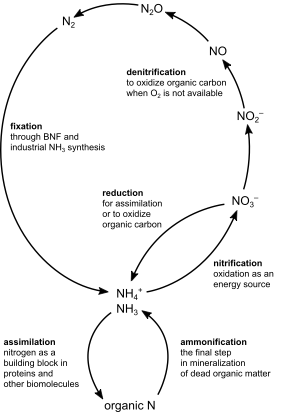
Moving onto soils, leaching and denitrification losses from agricultural land can be mitigated in many ways [46,136]. Since both nitrate (NO3−) leaching and denitrification are promoted by high NO3− concentrations and water flows (see Box 1), the most important mitigation measures build on ensuring low NO3− concentrations when crop demand is low and precipitation high. This is done, first and foremost, by applying N fertilizer in quantities and with timing adjusted to how much the crop can assimilate. Such approaches are often discussed under the heading ‘precision fertilization’ and the ‘4R’ approach (fertilizer application from the right source, at the right rate, right time, and right place). Another important technique is to ensure that something is growing on fields during rainy seasons, thereby keeping soil NO3− concentrations low. Perennial grasses on cropland and in permanent meadows and pastures generally have low leaching losses. In annual crop production, one common option in temperate climate is to grow winter-sown crops (e.g., winter wheat) or alternatively cover crops that grow during winter to absorb N that might otherwise be leached. These cover crops can then be used as feed or green manure, for example. Other approaches include the use of chemicals known as nitrification inhibitors, which slow the microbial conversion of ammoniacal N to NO3− and thereby can reduce losses through leaching and denitrification [158].
Losses of NH mainly occur in manure management and in connection to application of manure and synthetic fertilizers. There are several approaches to mitigate these losses [44–46]. NH3 emissions are driven by the chemical equilibrium between water-dissolved ammoniacal N ⇌ ammonia gas. Losses increase with increasing ammoniacal N concentrations, substrate pH, temperature, and wind speed. Therefore, key technologies build on reducing air contact time and wind speed, lowering temperature, lowering pH, and lowering ammoniacal N concentrations. For example, technologies include: covering manure storages; lowering temperature and ventilation of animal houses and manure storage; acidifying manure (lowering pH) in storage or at application using, e.g., sulphuric acid14; incorporating fertilizers into soil to reduce air contact; spreading manure on cool days if possible, ideally with a light rain to infiltrate N in soil; using chemicals known as urease inhibitors, which slow the microbial decomposition of urea to ammonium; and capturing NH3 from animal houses and manure storage using air scrubbers and other technologies. Since several of these mitigation technologies are relatively easy to standardize and evaluate, researchers have been able to quantify the potentials and costs of these measures relatively well [44–46,136].
Reducing food waste appears in many analyses to offer a considerable opportunity to reduce N waste throughout the agri-food system. The precise quantities, and even more the mitigation potential, are however notoriously difficult to quantify. It is clear that many crop and livestock products intended for human consumption are not ultimately ingested by humans for numerous reasons including processing waste, disqualification by quality standards, and spoilage during storage. But reliable primary data on food loss and waste are scarce, and often inadequate in detail to make reliable estimates of how much loss and waste could actually be avoided [11,161–165]. The United Nations Environment Programme (UNEP) has estimated that about 17% of global consumer food supply in 2019 was wasted (counted as fresh weight); it notes, however that this figure includes both edible and inedible parts and that reliable estimates are lacking of the edible share that could theoretically be avoided [165].
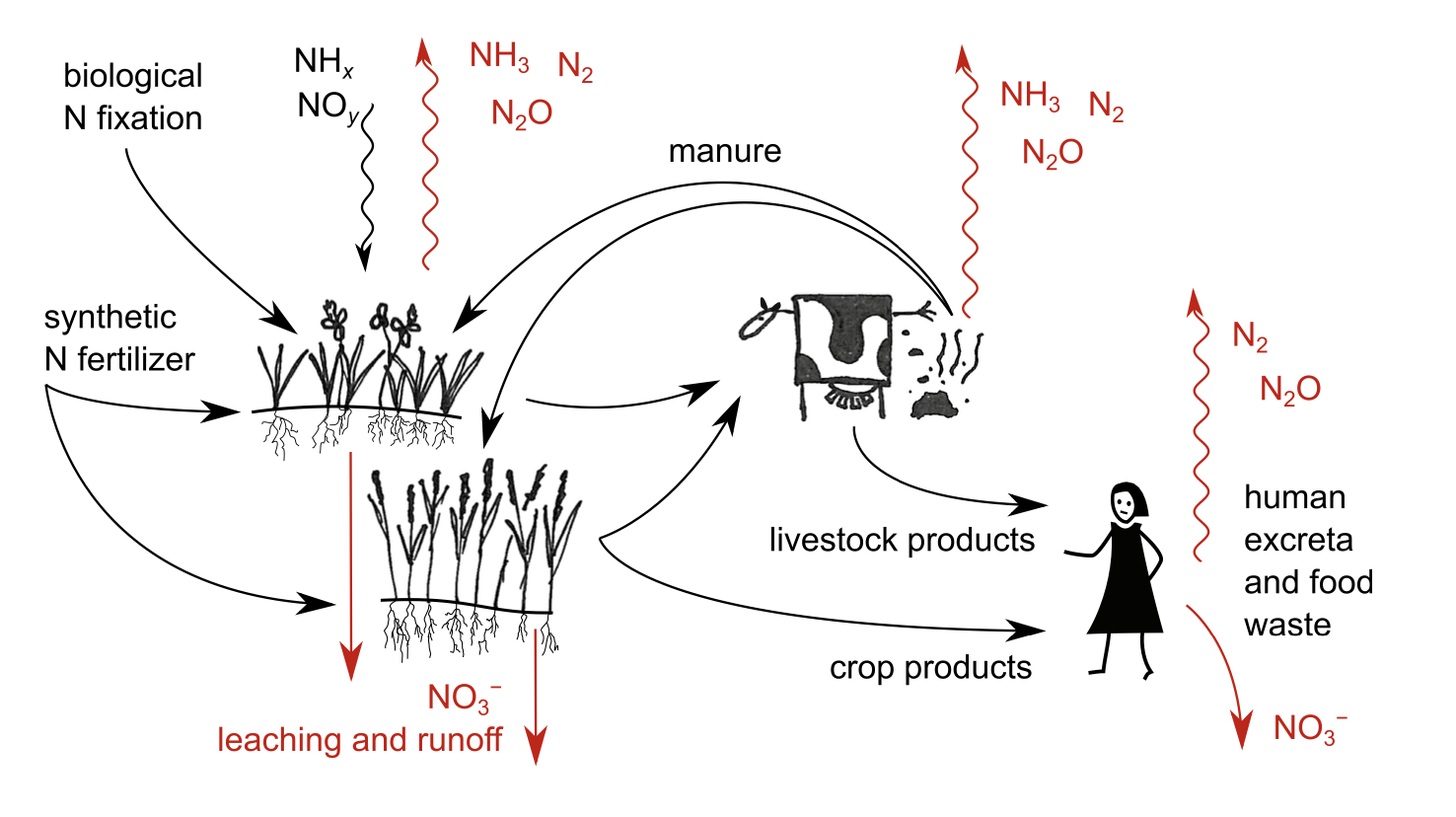
When it comes to increasing N circularity, the largest unused potential is undoubtedly human excreta. Human N excreta globally equal about one-quarter to one-third of total synthetic N use, but this resource is currently almost entirely wasted (see Section 3.2). There are many possible methods for hygienic and safe recovery of nutrients from human excreta [38], but, unfortunately, reaching a high degree of recovery would require huge infrastructure investments to move away from the present model for ‘modern’ waste treatment, which is based on wastewater systems and maximizing N2 denitrification in wastewater treatment plants [37–39]. Sewage and wastewater treatment systems are an enormous technical lock-in.
Food waste recycling is another opportunity for increased N circularity. As mentioned above, food waste quantities are difficult to estimate, and it is even more difficult to say what percentage of food loss and waste is already recycled as, e.g., livestock feed, or fertilizer through biogas or composting systems.
Moreover, some waste of livestock feed is inevitable in storage, processing, and in livestock housing. Feed waste in livestock houses in many cases ends up mixed into manure management systems, and so will have a similar recycling fate as manure N.
Inefficient recycling of livestock excreta is another issue that is difficult to quantify. Most livestock excreta are returned to agricultural land, either directly by grazing livestock on the land, or via storage and application of manure. A small share of livestock excreta (how much is highly uncertain but globally perhaps a few percent) is disposed of outside agriculture, e.g., used as fuel or discharged to the environment [40,166,167]. The largest inefficiency in manure recycling lies in the fact that some regions and farms have such high livestock concentrations that manure supply far exceeds crop nutrient demand [69]. Given the economic incentives to keep these high livestock concentrations, there is a growing interest in various technologies to produce concentrated N fertilizers from manure [168]. An alternative approach is to redistribute livestock to ensure that all manure can be efficiently used in crops; which brings us to the next point.
6.2 Change agriculture’s spatial distribution
Agriculture’s spatial distribution shapes environmental and health impacts in many ways. Broadly, there are two parts to this observation.
First, the spatial distribution of agricultural production determines its N use efficiency and emission intensity. A main reason for this is the diminishing marginal yield response to N inputs. Each additional unit of fertilizer has successively smaller effects on yields and thus causes successively higher N waste. Therefore, by spatially reallocating N inputs, perhaps between neighbouring farms, perhaps between continents, the overall crop yield will change depending on the different marginal yield responses [148]. A complicating factor is that yield response also depends on many other factors including soil types, climate, crop rotations, water limitation, and so on. There are also, of course, economic and legal constraints. In any case, research shows that the global distribution of agricultural production is far from optimal in terms of N use efficiency; or put another way: by prioritizing N input where it benefits crops the most, the same production could be achieved with far less N emitted to the environment [69,169–175]
Second, the spatial distribution of emissions determines their environmental and health impacts. A given amount of N pollution causes more harm in certain environments: particulate matter close to a population centre, atmospheric N deposition in a sensitive forest, or N leaching in a sensitive marine area, for example.
The spatial redistribution of agricultural production therefore affects both the quality and quantity of impacts. Research suggests that managing N inputs in light of these observations provides a major opportunity to maintain or even increase global food production while reducing environmental and health impacts [86,89,125,136,139,140].
In theory, this spatial redistribution idea holds an enormous potential to reduce damages to the environment and human health. But in practice, there is (luckily) no global central planner who is able to make such an optimization. Real-world implementation is mostly incremental and always imperfect, negotiated politically and economically through a patchwork of policies and market forces, and constrained by various practical factors. The theoretical explorations of this idea are nevertheless important, in order to map out the biophysical possibilities.
In practice, a large number of policies and market forces explicitly target the spatial structure of agriculture, although they are not always described as such, and they are not always implemented to the benefit of the environment. Examples of such policies and market forces include protected areas, subsidies and taxes, trade regulations, and agricultural intensity regulations. An important class of such policies are agricultural production subsidies, which shape production volumes and methods, unfortunately often in severely suboptimal ways from a societal viewpoint [176]. Another is market regulations, such as the former EU milk quota system, which had strong effects on intensity and spatial structure of milk production in the EU. A third class of examples are environmental policies, for example: policy support for organic agriculture (which has lower input and production intensity than conventional agriculture); the EU Nitrates Directive (1991), which requires member states to designate Nitrate Vulnerable Zones, where application of manure N is limited to maximum 170 kg N/hectare/ year [177]; and the EU Habitats Directive (1992) which is currently reshaping agriculture in the Netherlands after courts have ruled that the country is not doing enough to abate NH3 emissions considering biodiversity impacts [178].
6.3 Change human diets
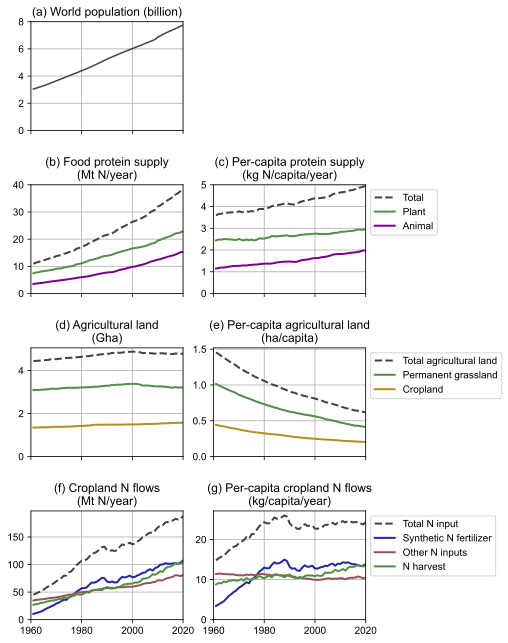
In principle, a dietary shift away from the most N-intensive foods offers a huge potential to reduce the environmental impacts of food production. Per-capita supply of animal-source protein varies enormously between countries, from almost zero to over 80 g/capita/day (see Figure 7). Globally, animal-source food provides about 40% of dietary protein. For some populations where there are serious problems of hunger and micronutrient deficiency [179], these animal-source foods can play an important role in improving human nutrition. However, in affluent contexts where intake of animal-source food is currently very high but where people can also afford and access a wide range of plant-based foods, the nutritional importance of animal-source food is much smaller [179]. As a global average, humans consume well above their dietary needs of most nutrients [180]. To reduce N emissions and related impacts, the most effective change would be for these high consuming populations to reduce their consumption of animal-source food (meat, dairy, and eggs). This would strongly reduce the total need for feed production, manure management and application, and related N emissions [149,181–183].
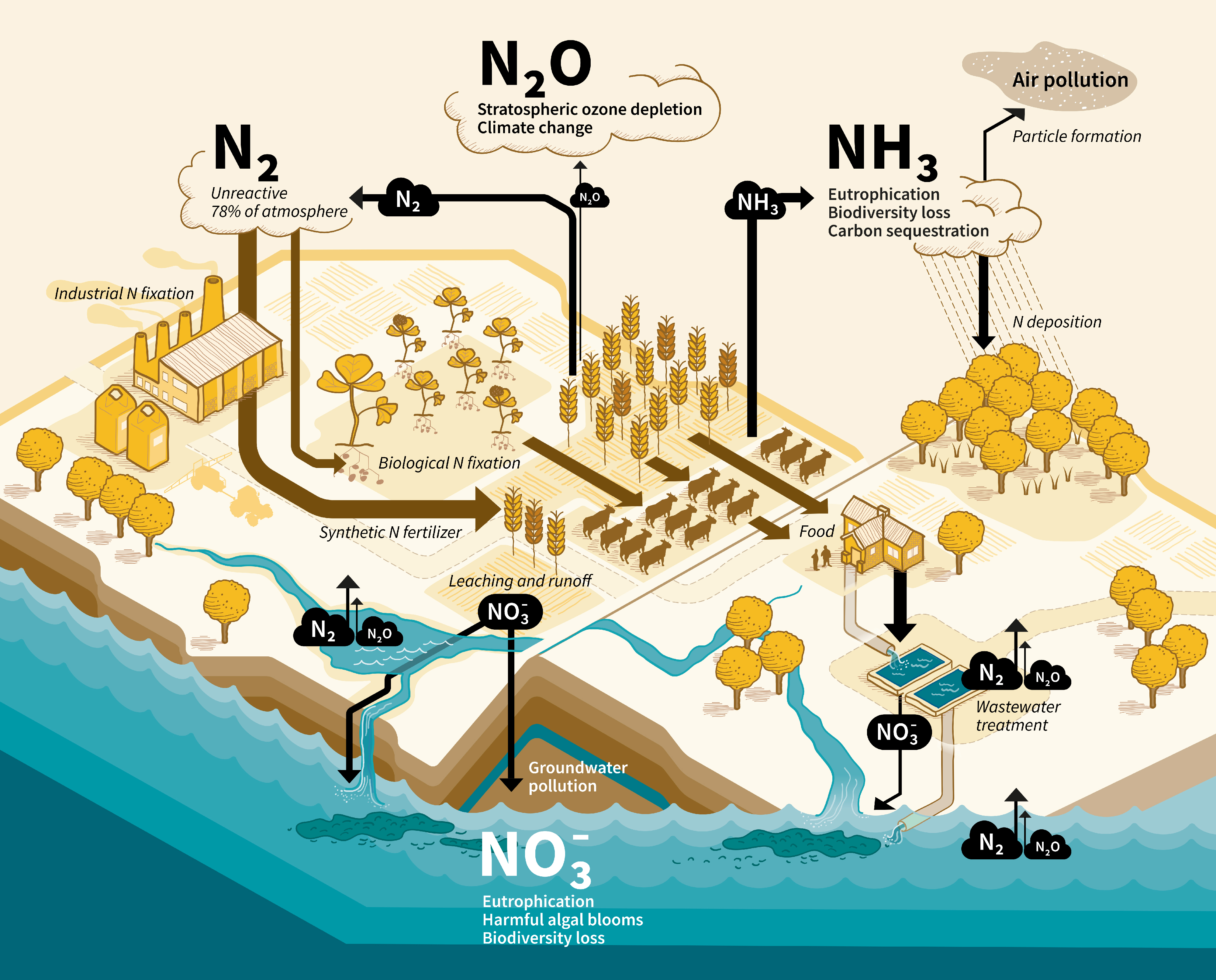
Figure 7: Per-capita food protein supply and gross domestic product (GDP) in countries of the world. Each marker represents one country, with size proportional to population. The dashed lines are linear regressions. The shaded band shows a range of minimum safe protein intake, calculated from the WHO safe protein intake (0.83 g protein/kg body weight/day) and the global range of country-average body weights (50–82 kg/capita) [184,185]. Data from the FAOSTAT database.
6.4 Summary of approaches and potentials
Research shows that an ambitious and integrated package of measures to increase efficiency and optimize agriculture’s spatial distribution could reduce future food-system N losses by 30–50%, without dietary change, compared to business-as-usual scenarios [136,148,149,171,175,182,183,186,187]. There are however several major uncertainties in any such estimate; and it must be noted that these are merely estimates of technical possibilities, not predictions of likely implementation.
Human dietary change offers an additional and very large potential to reduce N losses. Different scenarios for future human diets vary enormously in terms of N losses. As a point of reference, present-day food protein supply (from both plant and animal sources) varies between countries from less than 60 g/person/day to about 120 g/person/day. Almost all of this variation is in animal-sourced proteins, which have relatively high N emission intensity. The most affluent countries are therefore responsible for per-capita N losses several times higher than the poorest countries [188,189].
While humans can survive on very frugal diets consisting mainly of cereals or starchy roots, as do the poorest people in the world, such a scenario for the future is of limited interest since it is both unlikely and highly undesirable (both nutritionally and culturally). Rather, most serious scenario analyses of dietary shifts start from a baseline projection based on likely growth in population and affluence, and then explore various changes from that starting point. Such research shows that adoption of healthy but less N-polluting diets might plausibly cut future food-system N losses by 10–40% compared to business-as-usual scenarios [77,148,149,181–183,186,187]. With a combination of production and consumption side measures it is therefore clearly possible to cut global N emissions by 50% or more.

![Figure 4: Nitrogen (N) flows in the global agri-food system in 1961 and 2013, following the Generalized Representation of Agri-Food Systems [6,7]. The width of arrows correspond to the size of annual flows. Numbers are in million metric tonnes per year (Mt N/year). Arrows pointing out from agricultural land, livestock, and human population represent N losses including dinitrogen (N2). Diagram adapted from [7] in collaboration with Luis Lassaletta.](/sites/default/files/2024-02/Figure4.png)
![Figure 7: Per-capita food protein supply and gross domestic product (GDP) in countries of the world. Each marker represents one country, with size proportional to population. The dashed lines are linear regressions. The shaded band shows a range of minimum safe protein intake, calculated from the WHO safe protein intake (0.83 g protein/kg body weight/day) and the global range of country-average body weights (50–82 kg/capita) [184,185]. Data from the FAOSTAT database.](/sites/default/files/2024-02/Figure7.png)




Comments (0)