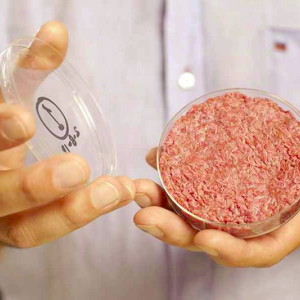
This article in Nature Scientific Reports details a new approach for generating skeletal muscle from pigs which can be used to make skeletal muscle – the main component of pork meat – in vitro. The techniques are potentially applicable to other types of muscle, such as heart muscle tissue, as well.
The study describes the steps the authors took to create muscle tissue which has the potential for indefinite expansion. Their approach, which includes working with pluripotent stem cells, has some major advantages over previous techniques which used somatic myogenic progenitors, which are stem cells which have already been designated as producers of muscle tissue. The latter have limited self-renewal properties, whereas the new approach promises continued reproduction.
Importantly, the authors showed that they could grow the tissue without ‘animal serum’, which is liquid derived from blood. Other lab-grown meats have needed this serum for their manufacture; which has been identified as a problem for commercialisation because it is hard to harvest and store, and because it continues the reliance on livestock to produce the meat.
The authors believe that with additional work, the approach can be adapted to other species and cell lines. The techniques were developed with a number of potential uses in mind, both medical and for food production. However, issues around mass production and commercialisation of any lab-grown meat product mean that it may take a while before cultured pork finds its way to our plates.
Abstract
The pig is recognized as a valuable model in biomedical research in addition to its agricultural importance. Here we describe a means for generating skeletal muscle efficiently from porcine induced pluripotent stem cells (piPSC) in vitro thereby providing a versatile platform for applications ranging from regenerative biology to the ex vivo cultivation of meat. The GSK3B inhibitor, CHIR99021 was employed to suppress apoptosis, elicit WNT signaling events and drive naïve-type piPSC along the mesoderm lineage, and, in combination with the DNA methylation inhibitor 5-aza-cytidine, to activate an early skeletal muscle transcription program. Terminal differentiation was then induced by activation of an ectopically expressed MYOD1. Myotubes, characterized by myofibril development and both spontaneous and stimuli-elicited excitation-contraction coupling cycles appeared within 11 days. Efficient lineage-specific differentiation was confirmed by uniform NCAM1 and myosin heavy chain expression. These results provide an approach for generating skeletal muscle that is potentially applicable to other pluripotent cell lines and to generating other forms of muscle.
Reference
Genovese, N.J., Domeier, T.L., Telugu, B.P.V. and Roberts, R.M., 2017. Enhanced Development of Skeletal Myotubes from Porcine Induced Pluripotent Stem Cells. Scientific Reports, 7, p.41833.
You can find the paper here (open access).
News coverage of the story, with a focus on its potential applications for livestock-free meat production here.







Post a new comment »